ophthafutur®
hpmc
Dispersive ophthalmic viscosurgical device (OVD) used to create and maintain anterior chamber depth and visibility, as well as to protect intraocular tissues during surgery.
- Cristal clear view
- Endotoxin free
- Preloaded syringe

What is HPMC?
Hydroxypropylmethylcellulose (HPMC) is a semi-synthetic cellulose-based polymer which is used as a thickening agent, an emulsifier and as a stabilizer in a variety of products including food, pharmaceuticals and cosmetics.
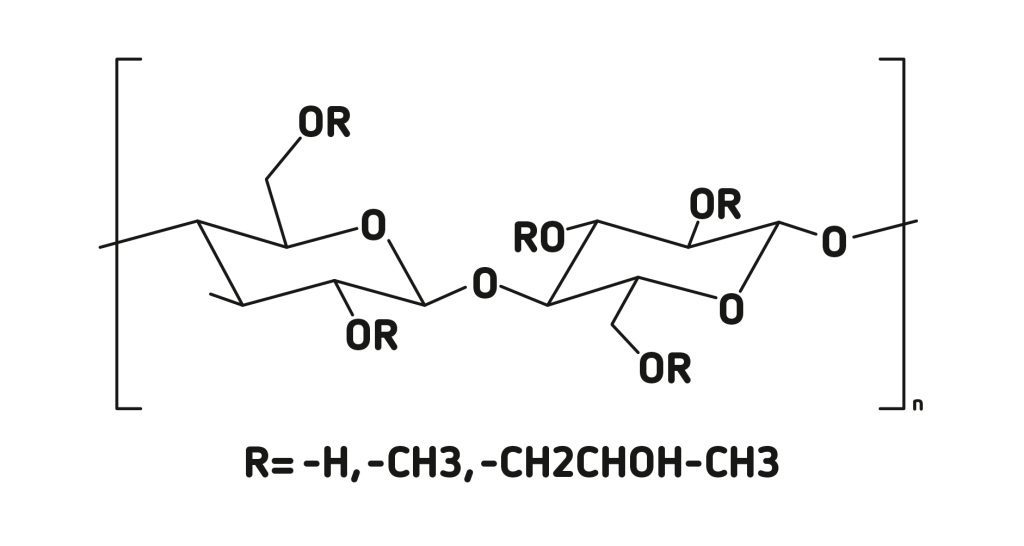
In ophthalmology it is used due to its good wetting properties, excellent tissue and endothelium protection, efficient volume replacement and quick removal. Specifically, it can be used to wet the cornea during cataract, corneal or retinal procedures.
The Challenge
Bacterial endotoxins and injectability
HPMC is made from natural cellulose and the appropriate removal of microbial impurities is a major manufacturing challenge.
The same applies to achieve the needed high viscosity together with an unobstructed (bubble-free) view through the media of the eye and an easy and simple injectability.
The Solution
Scientifically-based engineering

ophthafutur® hpmc complies with the standards for pharmaceutical products and is manufactured according to the relevant GMP guidelines in a fully controlled clean room environment.
Filling of the product under clean room class A conditions prevents any product contamination and the applied advanced vacuum filling technology allows for bubble-free products.
Usability
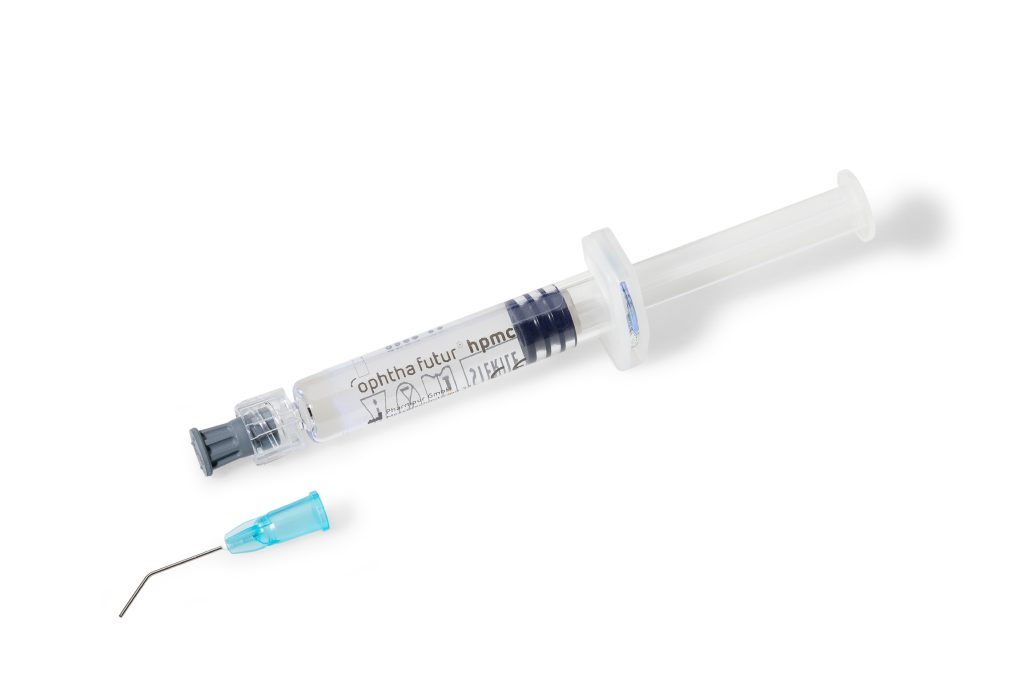
ophthafutur® hpmc is intended as an auxiliary during ophthalmologic interventions and examinations.
Intraocularly, ophthafutur® hpmc is used as a volume substitute for the aqueous humor to maintain anterior segment integrity and anatomical spaces during intraocular interventions, to protect intraocular tissues (i.e. the corneal endothelium), to sustain corneal transparency as well as for the lubrication of intraocular lenses (IOL) and surgical instruments.
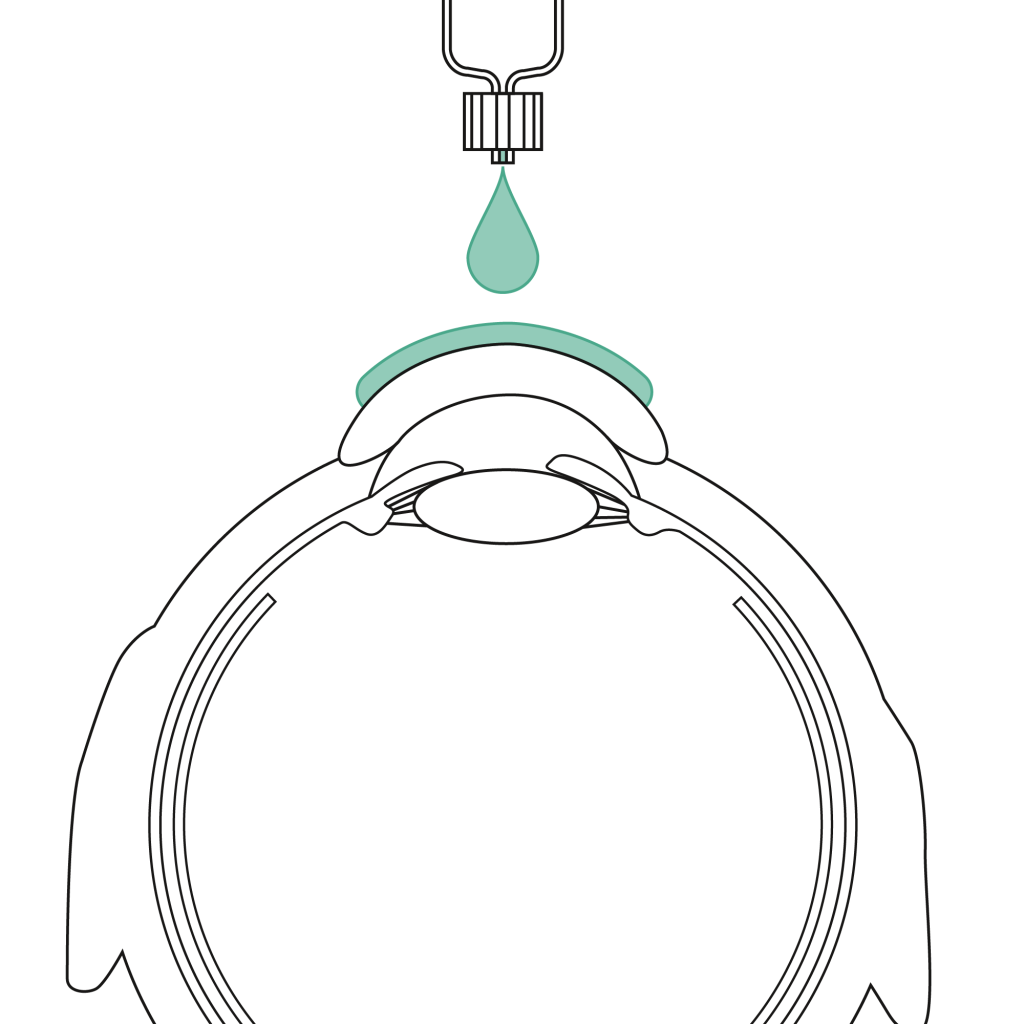
Extraocularly, ophthafutur® hpmc is used for the moisturization and protection of the corneal anterior surface by pre-corneal application throughout ophthalmologic interventions, or it is used as an adjuvant for diagnostic and therapeutic contact lenses. Additionally, ophthafutur® hpmc is used for lubrication of IOL implantation instruments (injectors).
For further in-depth information please contact your local distributor.



